FDA Investigator Mark R Mcclain
Mark R Mcclain has conducted inspections on 144 sites in 13 countries as of 13 Mar 2012. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
144
Last Inspection Date:
13 Mar 2012
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Ireland,
Japan,
Germany,
Sweden,
New Zealand,
France,
India,
China,
Canada,
Denmark,
Singapore,
Poland
FDA Investigators that have inspected at least one site in common with Mark R Mcclain:
Abby E Pelletier,
Adetutu M Gidado,
Alberto A Viciedo,
Alexander V Gontcharov,
Alice C Silva,
Alice S Tsao,
Alicia M Mozzachio,
Allison A Aldridge, PhD,
Alysia M Salonia,
Amanda E Lewin, PhD,
Amber G Wardwell,
Amy C Jordan,
Amy N Chen,
Anastasia M Shields,
Andrew M Barlow,
Anissa M Vargas,
Ann Marie Montemurro,
Anna Lazar,
Anne E Carpenter,
Anthony C Warchut,
Anthony N Onianwa,
Arsen Karapetyan,
Ashar P Parikh,
Asia D Clark,
Aurora Miha Trifanov,
Azza Talaat,
Barbara D Wright,
Barbara J Rincon,
Bernice F Hirsch,
Bijoy Panicker,
Blake C Hamann,
Bo Chi, PhD,
Bogdan Kurtyka,
Bradley D Newsad,
Brandy N Lepage,
Brenda P King,
Brenda W Uratani, PhD,
Brenna L Macdowell,
Bruce H Mccullough,
Burnell M Henry,
Byungja E Marciante,
Calvin B Koerner,
Camerson E Moore,
Carl A Huffman, III,
Carla A Norris,
Carla J Lundi,
Carlos Chavez,
Catherine J Laufmann,
Charanjeet Jassal,
Charisse K Green,
Charles I Ann,
Charles R Cote, RIC,
Christian D Lynch (CDL),
Christine H Lee,
Christine L Williams,
Christine M Whitby, CSO,
Christopher J Cruciotti,
Christopher Janik,
Chunsheng Cai, PhD,
Concepcion Cruz, Jr,
Constance Desimone,
Constantin Y Philopoulos,
Courtney N Long,
Cynthia F Kleppinger, MD,
Darin S Wiegers,
Daryl A Dewoskin,
Deborah A Greco,
Demitria J Xiradakis,
Dennis Cantellops Paite,
Diane C Thibodeau,
Diane L Raccasi,
Diane M Prince,
Dien N Nguyen,
Dominic R Frasca,
Donald C Obenhuber, PhD,
Dorota Matecka, PhD,
Dorothy P Kramer,
Douglas A Campbell,
Dr. Robert C Horan, MD,
Dr. Zhihao Qiu (Peter), PhD,
Edmund F Mrak, Jr,
Eduardo L Rodriguez,
Edward J Janik,
Eileen A Liu,
Elizabeth B Griffin,
Elizabeth M Jacobson,
Emily J Orban,
Emily J Schramm,
Eric C Schmitt,
Erin I Keith,
Evelyn Taha,
Felix Maldonado,
Frederick F Razzaghi,
Gabrielle J Swain,
Gale E Ryan,
George M Ibrahim,
George T Allen, Jr,
Gerard D Difiore,
Gwyn G Dickinson,
Hang N Guo,
Heika R Tait,
Helen B Ricalde,
Intkhab H Wahla,
Israel Santiago, OCM,
Jacek Cieslak, PhD,
Jacqueline Mdiaz Albertini,
James A Jagon,
James P Stumpff,
Janete F Guardia,
Jason A Rossi,
Jason F Chancey,
Jawaid Hamid,
Jeffery A Hangartner,
Jeffrey A Sommers,
Jeffrey J Thibodeau,
Joan A Loreng,
Joanne E King,
Joey V Quitania,
John A Gonzalez,
John A Hollings,
John J Thibodeau,
John P Mistler,
John P Shelton,
Jonah S Ufferfilge,
Jonathan G Matrisciano,
Jose Acruz Gonzalez,
José E Meléndez,
Jose Ohernandez Guzman,
Jose R Hernandez,
Jose R Lopez,
Joyce A Kellett,
Julie D Bringger,
Junho Pak,
Justin A Boyd,
Karen A Briggs,
Karen M Cooper,
Katherine Szestypalow,
Kathleen E Mcafoose,
Kenneth H Williams,
Kenneth Nieves,
Kent A Conforti,
Kevin P Foley,
Kham Phommachanh,
Kim Lthomas Cruse,
Kimberly M White,
Kristina J Donohue,
Kristy A Zielny,
Larry K Austin,
Lata C Mathew, PhD,
Laurie B Frazier,
Lawrence J Stringer,
LCDR Debra Emerson,
LCDR Michael H Tollon,
Lei Zhang, PhD,
Leiyun Boone, PhD,
Li Hongpaul Yeh, PhD,
Liming Zhang,
Linda F Murphy,
Linda L Green,
Lirissia Mccoy,
Lisa M Bellows,
Lisa R Hilliar,
Lixia Cai,
Lori M Newman,
Lorie S Hannappel,
Lucas B Leake,
Marcus F Yambot,
Margaret E Walsh,
Margaret M Annes,
Margaret M Sands,
Maria Joselopez Barragan, PhD,
Marianela Aponte Cruz,
Marie F Morin,
Martin J Guardia,
Mary Jeanet Mcgarry,
Maryam Tabatabaie,
Matthew B Casale,
Matthew C Watson,
Matthew D Silverman,
Maura Rooney,
Maya M Davis,
Mecado,
Megan A Haggerty,
Meisha A Mandal,
Meisha R Sampson,
Meisha Waters,
Miah Jung,
Michael A Charles,
Michael G Mayfield,
Michael P Sheehan,
Michael R Goga,
Michael Shanks, MS,
Michele L Obert,
Michelle M Noe Varga,
Michelle Yclark Stuart,
Miguel A Martinez Perez,
Mihaly S Ligmond,
Mikayla K Deardorff,
Mra Mcculloughj,
Mra Munizn,
Nabil Nakhoul,
Nancy G Schmidt,
Nancy L Rolli,
Nealie C Newberger,
Nicholas A Violand,
Nicholas F Lyons,
Nicholas L Paulin,
Nina Yang,
Omotunde O Osunsanmi,
Pal S Mayasandra,
Pamela N Agaba,
Paraluman S Leonin,
Parul M Patel,
Paul A Bonneau,
Paul E Stein,
Paul L Bellamy,
Paula A Trost,
Peter R Lenahan,
Philip F Istafanos, DMV, MS,
Philip Kreiter,
Prabhu P Raju,
Qin Xu,
Ramon E Martinez,
Rapti D Madurawe,
Raymond T Oji,
Rebecca B Welch,
Rebecca Rodriguez,
Regina T Brown,
Reginald D Neal,
Reyes Candau Chacon, PhD,
Richard H Penta,
Richard Ledwidge (nmi), PhD,
Robert D Tollefsen,
Robert J Ham,
Robert J Martin,
Robert Jennings,
Robert L Stevenson,
Robert O'brian,
Roger F Zabinski,
Rose Ashley,
Rumany C Penn, PharmD,
Russell J Glapion,
Russell K Riley,
Samir C Gala,
Samuel T Walker,
Sandra A Boyd,
Sandra A Hughes,
Sangeeta M Khurana, PhD,
Santos E Camara,
Sarah M Meng,
Sarah M Napier,
Sean R Marcsisin,
Sharon K Gershon,
Sharon K Thoma, PharmD,
Sherry G Bous,
Shirley J Berryman,
Sidney B Priesmeyer,
Simone E Pitts,
Stacey S Degarmo,
Stephanie Mangigian, MS/OSH, RN,
Stephen C Smith,
Stephen D Brown,
Stephen J Mottola,
Stephen R Souza,
Steven D Kehoe,
Steven Fong, MS, PhD,
Steven M Weinman,
Susan F Laska, MS,
Susan M Jackson,
Susan T Hadman,
Susanne M Richardson, MS RAC,
Suzanne M Healy,
Taichun Qin, PhD,
Tajah L Blackburn,
Tamil Arasu, PhD,
Tanya R Syffrard,
Temar Q Williams,
Thomas J Arista,
Thomas Siebertz,
Todd D Alspach,
Tomika L Crafter,
Tracey L Harris,
Troy R Petrillo,
Truong Xuan Nguyen (Andy),
Tyanna N Hadley,
Victoria M Daddeo,
Viviana Matta,
Walden H Lee,
Wayne E Seifert,
William Hallett, PhD,
William J Leonard,
Xiaohui Shen,
Xiaojun Yan,
Xikui Chen (nmi), PhD,
Yetao Jin, PhD,
Yiying E Chen,
Yumi J Hiramine,
Yvins Dezan,
Yvonne E Lozano,
Ziyang Su, PhD
Mark R Mcclain's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| August, 2004 | FDA 483 | Ecometics, Inc. - Form 483, 2004-08-02 |
| December, 2003 | EIR | Eli Lilly And Company - EIR, 2003-12-16 |
| September, 2006 | FDA 483 | CooperSurgical, Inc. - Form 483, 2006-11-06 |
| August, 2006 | FDA 483 | Phoenix Divina Products Llc - Form 483, 2006-08-15 |
| December, 2007 | FDA 483 | Alexion Pharmaceuticals, Inc. - Form 483, 2007-12-19 |
| May, 2008 | FDA 483 | Sheffield Pharmaceuticals LLC - Form 483, 2008-05-15 |
| December, 2011 | FDA 483 | Serumwerk Bernburg AG - Form 483, 2011-12-01 |
| December, 2009 | FDA 483 | Mannkind Corporation - Form 483, 2009-12-04 |
| December, 2008 | EIR | Charles River Laboratories Ireland Ltd. - EIR, 2014-05-08 |
| April, 2010 | FDA 483 | RHG & Company Inc. - Form 483, 2010-04-23 |
| June, 2006 | FDA 483 | Computerx Phamacy, Inc. - Form 483, 2006-06-09 |
| December, 2008 | FDA 483 | LEO Laboratories Ltd. - Form 483, 2008-12-08 |
| January, 2003 | EIR | Pfizer Laboratories Div Pfizer Inc - EIR, 2003-01-23 |
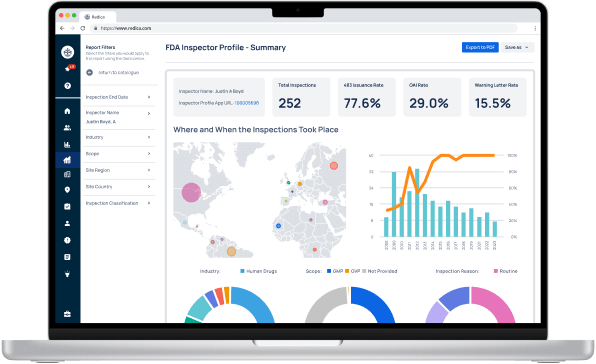
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more