FDA Investigator Jessica J Lee
Jessica J Lee has conducted inspections on 18 sites in 1 countries as of 15 May 2023. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
18
Last Inspection Date:
15 May 2023
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America
FDA Investigators that have inspected at least one site in common with Jessica J Lee:
Alice A Blair,
Brandon R Boone,
Bruce D Broidy,
Christopher Do,
Clarice J Ahn,
Courtney R Bolton,
Daniel J Munoz,
David A Quale,
Edna J Hidalgo,
Emal Wahab,
Emily Chang,
Eric J Ferguson,
Gena M Mckahan,
James S Lo,
Janice Wai,
Jeanne A Young,
Jeannie Pradyanata,
Jeffrey W Shrifter, DPM,
Jocelyn T Ramos,
Juan A Morales,
Kimberly L Anderson,
Larkin R Buckalew,
Lorraine N Williams,
Makia P Dove,
Marissa J Chan,
Mark I Nakano,
Melody M Ragasa Leong,
Misty L Treece,
Nicholas J Lahey,
Nympha V Florendo,
Randall J Plunkett,
Ronald P Boyce,
Steve P Yost,
Steven B Chiu,
Thomas N Adjabeng,
Travelle D Mason,
Wayne L Jefferson,
William J Weis
Jessica J Lee's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| March, 2020 | EIR | Purcell International - EIR, 2020-03-17 |
Experience Redica Systems’ NEW investigator profiles and dashboards
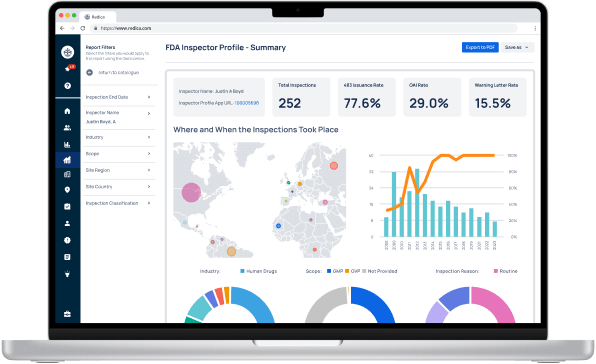
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more