FDA Investigator Willy A Orantes
Willy A Orantes has conducted inspections on 243 sites in 1 countries as of 20 Jun 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
243
Last Inspection Date:
20 Jun 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America
FDA Investigators that have inspected at least one site in common with Willy A Orantes:
Aaron J Adler,
Addie V Scott Boston,
Alberto A Viciedo,
Alejandra Mcclellan,
Ali A Patino,
Alice S Tsao,
Allen Lou,
Alois P Provost,
Althea A Williams,
Amber G Chung,
Amy Kim,
Amy L Singer,
Amy M Cramer,
Andrew F Cohen,
Andrew J Molesky,
Anita R Michael,
Anna M Alexander,
Anthony M Criscuolo, Jr,
Anthony R Rauert,
Anthony W Lee,
Anthony W Thomas,
Ashley Defilippis,
Ashley Polizzotto,
Atul J Agrawal,
Austin B Appler,
Ayorinde O Babalola,
Barbara A Gullick,
Barbara J Holladay,
Barbara J Maulfair,
Benjamin Bustamante,
Betty M Ng Lee,
Brian M Brodman,
Brittney Hu,
Cary Greene,
Cassandra Gasby,
Charisse K Green,
Christian Parra,
Christine M Cerenzio,
Christine M Rivera,
Christopher S Fields,
Coleburn E Vielehr,
Cordero T Kimbrell,
Cosmon L Barrett,
Craig W Swanson,
Daniel Green,
David P Rice, Jr,
David R Delucia,
Deep R Parikh,
Denise L Terzian,
Devina K Budhan,
Devon O Seymour,
Dhaval H Patel,
Diane B Radice,
Dipesh K Shah,
Donald J Ullstrom,
Donald L Lech,
Donyel M Jones,
Doreen P Gubbay,
Douglas G Ng,
Edward D Mcdonald,
Edwin A Evangelista,
Emest F Bizjak,
Emilio Z Azukwu,
Eric J Cunningham,
Eric Rothschild,
Erica B Raphael,
Erin D Mccaffery,
Feryal Ahmad,
Frank A Boardwick,
Frank J Koch,
Frank Verni,
Gabriel Feng,
Gale L Glinecki,
Gayle S Lawson,
Gianine E Delade,
Gianine E Tompkins,
Hala L Selby,
Heath R Harley,
Hector Jcolon Torres,
Helen Verdel,
Hitesh R Patel,
Irina Gaberman,
James T Mccoy,
Jamie D Swann,
Jana J Lau,
Jananiga Vanniyasingam,
Janaysia N Trail,
Jane C Chen,
Jason Kwong,
Jason M Sluzynski,
Jean M Kelahan,
Jeffrey A Reed,
Jeffrey J Ebersole,
Jennifer Macmillan,
Jesse N Nardone,
Jesse Ray N Nardone,
Jessica D Nanini,
Jo Annc Declement,
Joel R Merriman,
John A Hollings,
John Dan,
John E Aghadia,
Johnmartin Jackson,
Joseph Duca,
Joseph H Procopio, Jr,
Joseph S Fanelli,
Joy F Canlas,
Judy Keihl,
Juliana Falla,
Julianna M Chedid,
Justin C Fuller,
Justina A Vicioso,
Karen A Spencer,
Karen E D'orazio,
Karen F Tomaziefski,
Katrina M D'egidio,
Keith M Reilly,
Kelli F Dobilas,
Kelly Doremus,
Kenneth Nieves,
Kerry A Kurdilla,
Kevin J Flessa,
Kim G Campbell,
Kimberley A Ricketts,
Kimberly A Coward,
Kristen C Jackson,
Kristina J Donohue,
Kristy A Zielny,
Kwong P Lee,
Laishan L Lam,
Larry Johnson,
Lawrence R Johnson,
Leticia S Jones,
Linda J Eason,
Loretta J Miller,
Lourdes A Faria Feldman,
LTJG Matthew R Palo,
Marjorie D Schultz,
Martha Sullivan Myrick,
Matthew A Spataro,
Matthew M Henciak,
Maximillian Lucci,
Melissa B Libby,
Melissa N Gregorio,
Melka F Argaw,
Meredith L Sheridan,
Meredith P Soehl, Investigator,
Michael R Dominick,
Michael Serrano,
Michele C Murray Schrade,
Michelle E Noonan Smith,
Milan S Kunajukr,
Milan S Mcgorty,
Mildred L Mccray,
Mohamad A Chahine,
Morgan J Matthews,
Mouhamed H Halwani,
Myra L Gemmill,
Myra L Reed,
Nazmul Hassan,
Nicholas A Violand,
Nicholas E Squillante,
Nicholas J Morici,
Nicholas P Schaub,
Niketa Patel,
Nina D Patel,
Normica G Facey,
Omolola A Shola Philips,
Onieka T Carpenter,
Pasquale L Leone,
Pearl L Gonzalez,
Perez,
Peter M Trunk,
Peter R Caparelli,
Peter R Lenahan,
Phillip O Mathis,
Rachael A Moss,
Rachel E Wapniak,
Randolph Campos Santiago,
Raymond L Cheung,
Raymond Liu,
Raymond M Lam,
Regina Gibson Melton,
Richard D Manney,
Rita F Monfort,
Robert C Howard,
Robert E Moncayo,
Ronald Ifraimov,
Ronald T Colucci,
Ronald T Nowalk,
Russell J Glapion,
Samuel A Francis,
Sarah A Meehan,
Sarah K Graham,
Schultz,
Seemay A Lee,
Shantel S Brown,
Sharon Ferraro,
Sherri J Liu,
Shirley S Wen,
Shreya Shah,
Slater K Bartlett,
Sony Mathews,
Spiridoula Dimopoulos,
Stephanie M Roberts Pedotti,
Stephanie T Durso,
Sunita Vij,
Susan J Essenmacher,
Suwaskie S Smith,
Taina Pierre Pierre,
Tanvir Kaium,
Tara K Carmody,
Tara K Normandin,
Teresa L Fox,
Tonia F Bernard,
Tracy M Portelli,
Tressa T Lewis,
Vaishali J Patel,
Valeria A Moore,
Valerie C Reed,
Vincent T Duncan,
Wai H Lam,
Wayne J Meyer,
Weston K Szymanski,
William Leung,
William V Thompson,
Wilmarie Marrero Santiago,
Yuyan Liang,
Zakaria I Ganiyu,
Zameer Farouk
Willy A Orantes's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| May, 2023 | FDA 483 | Pet Proteins LLC DBA Roam Pets - Form 483, 2023-05-03 |
| August, 2020 | FDA 483 | Apollo Greek Foods & Pastries, Inc. dba DC Oriental Wholesaler - Form 483, 2020-08-12 |
| November, 2018 | FDA 483 | Banyan International LLC - Form 483, 2018-11-14 |
| May, 2024 | FDA 483 | Source One International - Form 483, 2024-05-15 |
| March, 2025 | FDA 483 | Pet Proteins LLC DBA Roam Pets - Form 483, 2025-03-13 |
| August, 2025 | FDA 483 | IBSA PHARMA INC - Form 483, 2025-08-07 |
Experience Redica Systems’ NEW investigator profiles and dashboards
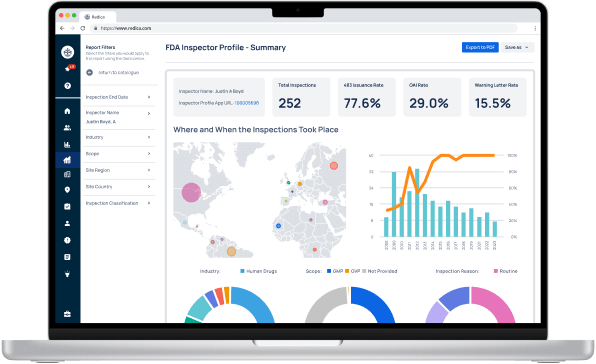
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more