FDA Investigator David A Oluwo
David A Oluwo has conducted inspections on 58 sites in 4 countries as of 13 May 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
58
Last Inspection Date:
13 May 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Canada,
Finland,
Switzerland
FDA Investigators that have inspected at least one site in common with David A Oluwo:
Abby E Pelletier,
Adetutu M Gidado,
Alberto A Viciedo,
Alice S Tsao,
Alison N Correll,
Alison N Stieg,
Amit Kokate,
Amy N Chen,
Anastasia I Offordile,
Anastasia M Shields,
Anita R Michael,
Anitha Palamakula Govada,
Ankur C Patel,
Anna Lazar,
Annemarie Bodnar,
Anthony A Charity,
Anthony F Lorenzo,
Anthony J Ladner,
Anthony W Hite,
Audrey Thereset Uy,
Azeezat M Lawal,
Azza Talaat,
Barbara A Gullick,
Barbara Jwilimczyk Macri,
Bijoy Panicker,
Brandi L Garbutt,
Brian D Young,
Brian R Yaun,
Brittany L Carpenter,
Brooke K Higgins,
Brooke K Seeman,
Bryan A Galvez,
Bryan J Love,
Burnell M Henry,
Candice C Mandera,
Candice J Cortes,
Carrie L Doupnik,
CDR Ileana Barreto Pettit,
Chaltu Nwakijra,
Charanjeet Jassal,
Chiang Syin, PhD,
Christian D Lynch (CDL),
Christian F Gomez Lugo,
Christina K Theodorou,
Christine M O'leary,
Christopher D Leach,
Colleen M Damon,
Concepcion Cruz, Jr,
Craig D Zagata,
Cynthia A Harris, MD, RN,
Cynthia A Palmer,
Cynthia J Lee, MS,
Cynthia White,
Daniel J Deciero,
Daniel J Grabicki,
Daniel J Min,
Daniel J Roberts,
Daniel L Zheng,
Daniel W Johnson,
David J Hafner,
David L Chon,
Deborah J Parris,
Debra L Pagano,
Dennis L Doupnik,
Derek S Dealy,
Devon Jenkins,
Deyaa Shaheen,
Diane T Bargo,
Douglas A Campbell,
Douglas C Kovacs,
Dr. Dominick Roselle,
Dr. Gang Wang, PhD,
Dr. Roslyn F Powers,
Dr. Yubing Tang,
Edmund F Mrak, Jr,
Edward D Mcdonald,
Edwin L Hobbs,
Emest F Bizjak,
Emmanuel Jramos Maldonado,
Eric L Dong, BS,
Erik T Nagaoka,
Evan Tong,
Fred Razzaghi,
Freddy Ortiz Colon,
Frederick F Razzaghi,
Gale L Glinecki,
Gayle S Lawson,
George Pyramides,
Gerald B Seaborn, Jr,
Ginger M Sykes,
Gwyn G Dickinson,
H Ljamilla Selby,
Hala L Selby,
Hector Jcolon Torres,
Helen Verdel,
Himanshu Gupta, PhD,
Iqra Iftikhar,
Jacqueline Mdiaz Albertini,
Jai P Singh,
Jaison J Eapen,
James M Mason,
James M Simpson,
James P Mcevoy,
James R Birkenstamm,
James W Leonette,
Janet A Rajan,
Jay B Shah,
Jay T Wong,
Jeanne Fringer, PhD,
Jeen S Min,
Jeffery A Hangartner,
Jessica L Pressley,
Joel D Hustedt,
Joey C West,
Jogy George,
John D Mcintosh,
John Dan,
Johnny D Nwankwo,
Jonah S Ufferfilge,
Jonathan G Matrisciano,
Jonathan W Chapman,
Jose Acruz Gonzalez,
José E Meléndez,
Jose R Hernandez,
Joseph T Dougherty,
Joseph W Matthews,
Joshua P Wireman,
Joy Rkozlowski Klena,
Juanita P Versace,
Julianne C Mccullough,
Junho Pak,
Justin A Boyd,
Justine Tomasso,
Karen A Briggs,
Karen E D'orazio,
Karishma G Gopaul,
Karyn M Campbell,
Katherine D Adams,
Kathleen M Jordan,
Kelly N Kerr,
Kendra L Brooks,
Kenneth H Williams,
Kenneth M Gordon,
Kenneth Nieves,
Kent C Faul,
Kevin A Gonzalez,
Kevin D Kallander,
Ko U Min,
Kristen D Evans,
Kristina J Donohue,
Kristina L Conroy,
Kristina Mjoyce Pittman,
Krystyna M Kitson,
Kyle S Hobson,
Lata C Mathew, PhD,
Latoya S Oliver Powell,
Lauren L Vajo,
Lauren N Barber,
Lawrence Harmon, Jr,
Lawrence J Stringer,
LCDR Debra Emerson,
LCDR Margaret Edi Gennaro,
LCDR Matthew R Mcnew, MPH, REHS/RS,
LCDR Susan E Polifko,
LCDR Wilfred A Darang,
Li Lu, PhD,
Linda K Cline,
Linda M Hoover,
Lisa B Orr,
Lisa Shin,
Logan T Williams,
Loretta Nemchik,
Lori M Newman,
Lori S Lawless,
LTJG Bradley E Benasutti,
Luis A Dasta,
Lydia I Rosas Marty,
Marc S Neubauer,
Marcellinus D Dordunoo,
Marcelo O Mangalindan, Jr,
Marcus A Ray,
Marea K Harmon,
Margaret Faulkner,
Margaret M Doherty,
Margaret M Sands,
Maria A Reed,
Maria Estrella,
Matthew M Henciak,
Matthew R Noonan,
Maureen A Wentzel,
Melissa J Garcia,
Melissa T Roy,
Meyer J Slobotsky,
Michael A Charles,
Michael Gurbarg,
Michael L Casner,
Michael O Idowu,
Michael P Sheehan,
Michael R Goga,
Michael R Klapal,
Michael Shanks, MS,
Michele Gottshall,
Mihaly S Ligmond,
Mindy M Chou,
Monique Rac Lester,
Nadeem I Chaudhry,
Namita Kothary,
Nancy A Saxenian Emmons,
Nancy F Scheraga,
Nancy L Rolli,
Nancy M Espinal,
Natasha Gupta,
Nathan B Davis,
Nawab A Siddiqui,
Nebil A Oumer,
Nelson N Ayangho,
Nicholas A Violand,
Nicholas L Paulin,
Niketa Patel,
Nikisha M Bolden,
Odera I Ekwunife,
Olumide A Akinyemi,
Parul M Patel,
Paul L Bellamy,
Pratik S Upadhyay, DDC,
Puspa Suresh Jayasekara,
Qin Xu,
Rachael O Oyewole,
Rachel C Harrington,
Rafeeq A Habeeb,
Raffi B Papazian,
Rebecca Rodriguez,
Robert B Shibuya, MD,
Robert H Wittorf,
Robert J Maffei,
Robert J Martin,
Robin Levis, PhD,
Rochelle L Cross,
Rosario D'costa,
Rose Ljean Mary,
Russell J Glapion,
Saleem A Akhtar,
Sam Pepe,
Samina S Khan,
Sandra A Boyd,
Sean R Marcsisin,
Sena G Dissmeyer,
Sharon K Thoma, PharmD,
Shirley S Wen,
Shirshendu K Deb, PhD,
Simone E Pitts,
Srinivas R Chennamaneni, PhD,
Stacey A Priest,
Stephanie L Shapley,
Stephanie Mangigian, MS/OSH, RN,
Stephen D Eich,
Steven E Kane,
Steven J Thurber,
Steven M Weinman,
Steven P Donald,
Suchan Kim,
Susan F Laska, MS,
Susan M Jackson,
Susanna E Ford,
Suzanne M Healy,
Suzanne M Muchene,
Swati Verma,
Tajah L Blackburn,
Tamil Arasu, PhD,
Tammy L Chavis,
Tammy M Phillips,
Tanya R Syffrard,
Tekalign Wondimu,
Temar Q Williams,
Terri L Dodds,
Thai D Truong,
Thomas E Friel,
Thomas J Arista,
Thomas R Withers,
Tiffani D Wilson,
Tiki J Dixon,
Timothy T Kapsala,
Tonia F Bernard,
Toyin B Oladimeji,
Tyanna N Hadley,
Unnee Ranjan,
Veronica Fuentes, MS,
Viviana Matta,
Vivin George,
Vlada Matusovsky,
Walden H Lee,
Walter L Fava,
Wanda Y Honeyblue,
William H Linkroum,
Xiandong Meng,
Yaharn Su,
Yan Qin,
Yong Wu, PhD,
Yoriann M Cabrera Bartolomei,
Young M Yoon,
Yumi J Hiramine,
Yvesna C Blaise,
Yvins Dezan,
Zachary A Bogorad,
Zhong Li, PhD,
Zhongren Wu,
Zi Qiang Gu
David A Oluwo's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
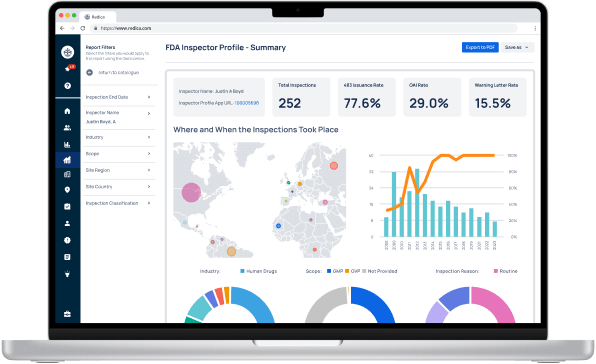
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more