FDA Investigator Michael Rosner
Michael Rosner has conducted inspections on 245 sites in 5 countries as of 21 May 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
245
Last Inspection Date:
21 May 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Spain,
Italy,
Mexico,
Switzerland
FDA Investigators that have inspected at least one site in common with Michael Rosner:
Abram A Brown,
Albert M Brindle,
Amber G Chung,
Andrew T Todd,
Anita R Michael,
Annemarie Bodnar,
Anthony M Criscuolo, Jr,
Antoinette L Ravelli,
Audrey Thereset Uy,
Barbara J Maulfair,
Barbara Jwilimczyk Macri,
Brandi L Garbutt,
Brandon J Brookens,
Brian D Young,
Brian R Yaun,
Brian S Keefer,
Byungja E Marciante,
Cara M Minelli,
Carol A Perry,
Carol A Robles,
Cary Greene,
Crayton Lee,
Crystal A Harlan,
Cynthia White,
Daniel W Johnson,
Deborah G Haney Cozzone,
Deborah H Cozzone,
Deborah J Parris,
Dena M Elimam,
Derek S Dealy,
Dessa A Antoine,
Devon Jenkins,
Dhaval H Patel,
Donyel M Jones,
Doreen P Gubbay,
Edward D Mcdonald,
Edward O'shaughnessy,
Edwin L Hobbs,
Emmanuel Obulor,
Eric Rothschild,
Eric S Myskowski,
Erika M Wilkerson,
Frank J Marciniak,
Gerald W Kopp,
Gianine E Delade,
Gillian B Nardelli,
Glenn S Quintanilla,
Gobiga Vanniyasingam,
Hitesh R Patel,
Howard B Rabinovitch,
Jacqueline A O'shaughnessy, PhD,
James P Mcevoy,
Janaye A Young,
Jeannie R Mayberry,
Jeen S Min,
Jennifer A Bazergui,
Jennifer Macmillan,
Jesse Ray N Nardone,
Jessica M Monteiro,
Jill M Sooter,
Jill Mielziner,
Jo Annc Declement,
Joseph L Despins, PhD,
Joseph R Hatcher,
Joseph S Fanelli,
Joseph T Dougherty,
Joy Rkozlowski Klena,
Judith A Jones,
Judith A Paterson,
Julianne C Mccullough,
Justin B Holder,
Justine Tomasso,
Karen F Tomaziefski,
Keith A Schwartz,
Kelly I Anderson,
Kendra L Brooks,
Kibily D Dembele,
Kim G Campbell,
Kris K Moore,
Kristen E Rescigno,
Krystyna M Kitson,
Kyle D Covill,
Kyle S Hobson,
Lata C Mathew, PhD,
Lauren L Vajo,
Lawrence R Johnson,
LCDR Wilfred A Darang,
Linda M Hoover,
Linda M Ross,
Lisa B Hall,
Lisa Harlan,
Loretta Nemchik,
LT John M Mastalski,
Marc A Jackson, Jr,
Marcelo O Mangalindan, Jr,
Margaret M Sands,
Martha Sullivan Myrick,
Matthew R Noonan,
Maureen A Wentzel,
Melissa B Libby,
Melka F Argaw,
Meredith L Sheridan,
Michael A Taylor,
Michael L Casner,
Nerizza B Guerin,
Nikisha M Bolden,
Pamela M Tremarki,
Paterson,
Ramanda C Gregory,
Raymond Cheung,
Raymond L Cheung,
Raymond M Lam,
Regina T Brown,
Remache,
Rene M Amaguana,
Richard D Manney,
Richard G Fochesato,
Robert D Ogan,
Robert T Lorenz,
Robert W Calais,
Robert W Mccullough,
Ronald T Colucci,
Russell J Glapion,
Russell P Vandermark,
Sarah J Park,
Sarah K Lemin,
Schultz,
Schwartz,
Sean D Duke,
Seng Ching Hwang,
Sherri J Liu,
Shirley S Wen,
Stephanie T Durso,
Stephen J Mottola,
Susan M Halsted,
Tamil Arasu, PhD,
Tegan M Vanartsdalen,
Teigan M Mule,
Todd J Maushart,
Tonya O Corbin,
Tressa T Lewis,
Valerie C Reed,
Wayne J Meyer,
Whitney S Reynolds,
William J Muszynski,
William W Dotts,
Yessika Mashinski
Michael Rosner's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| August, 2017 | FDA 483 | Safeway Fresh Foods LLC - Form 483, 2017-08-28 |
| November, 2010 | FDA 483 | Gaspari Nutra LLC - Form 483, 2010-11-01 |
| December, 2012 | EIR | Gaspari Nutra LLC - EIR, 2012-12-19 |
| June, 2011 | EIR | Gaspari Nutra LLC - EIR, 2011-06-06 |
| November, 2010 | EIR | Gaspari Nutra LLC - EIR, 2010-11-01 |
| February, 2017 | EIR | Bravo Packing, Inc. - EIR, 2017-02-22 |
| June, 2017 | EIR | Bravo Packing, Inc. - EIR, 2017-06-28 |
| January, 2016 | EIR | Bravo Packing, Inc. - EIR, 2016-01-28 |
| April, 2013 | EIR | Bravo Packing, Inc. - EIR, 2013-04-02 |
| July, 2018 | EIR | Bravo Packing, Inc. - EIR, 2018-07-18 |
| April, 2014 | EIR | Bravo Packing, Inc. - EIR, 2014-04-16 |
| August, 2022 | FDA 483 | Bravo Packing, Inc. - Form 483, 2022-08-05 |
Experience Redica Systems’ NEW investigator profiles and dashboards
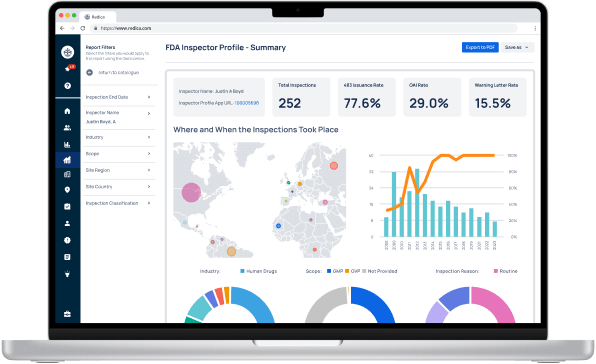
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more