FDA Investigator Reba A Gates
Reba A Gates has conducted inspections on 65 sites in 12 countries as of 22 Jul 2019. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
65
Last Inspection Date:
22 Jul 2019
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
China,
France,
United States of America,
Mexico,
Ireland,
Indonesia,
Canada,
India,
Japan,
Malaysia,
Italy,
Hong Kong
FDA Investigators that have inspected at least one site in common with Reba A Gates:
Aaron P Wozniak,
Adam R Cooke,
Adetutu M Gidado,
Alanna L Mussawwir Bias,
Alicia M Mozzachio,
Alphonso A Haupt, III,
Althea A Williams,
Ana P Pineda Zavaleta,
Andrea A Branche,
Angela E Glenn,
Anissa M Vargas,
Anita R Michael,
Anjali Shukla, PhD,
Ankur C Patel,
Anna Lazar,
Anna M Brannen,
Annet R Rajan,
Anthony A Charity,
Anthony C Warchut,
Anthony J Donato,
Anthony J Grace,
Arlyss M Jones,
Barbara M Frazier,
Betsy C Galliher,
Bichsa T Tran,
Bijoy Panicker,
Bonita S Chester,
Bonnie E Conley,
Brandon C Heitmeier,
Brandy D Brown,
Brandy N Lepage,
Brooke K Higgins,
Brooke K Seeman,
Bryan L Mcguckin,
Burnell M Henry,
Calvin Foulks,
Camerson E Moore,
Carl A Huffman, III,
Carl Lee,
Carlos A Ortiz,
CDR Thomas R Berry, PPh,
Charanjeet Jassal,
Charles M Edwards,
Chiang Syin, PhD,
Chris Alexander,
Christopher S Keating,
Claire M Minden,
Claudia Mperez Kasmarski,
Constance Y Fears,
Constantin Y Philopoulos,
Cynthia A Palmer,
Cynthia T Cain,
Dale A Nyberg,
Daniel J Roberts,
David M Beltran,
Davis,
Debra J Tucker,
Demitria J Xiradakis,
Dennis Cantellops Paite,
Devaughn Edwards,
Deyaa Shaheen,
Dipesh K Shah,
Doan T Nguyen, PharmD,
Dongping Dai, PhD,
Dr. Jason R Caballero,
Dr. Robert C Horan, MD,
Edmund F Mrak, Jr,
Edward H Maticka,
Eileen J Bannerman,
Ephrem Hunde, PhD,
Felix Maldonado,
Frank Wackes,
Gabriel Davila,
Gabriel R Mclemore,
George J Flynn,
George Pyramides,
Geraldine D Sanders,
German Rivera,
Grace E Mcnally,
Gregson A Joseph,
Gretchen M Laws,
Hala L Selby,
Hasan A Irier, PhD,
Heika R Tait,
Helen B Ricalde,
Helen Verdel,
Heriberto Negron Rivera,
Ibad U Khan,
Iris C Macinnes,
Ivy E Sweeney,
J'maica J Hunter,
Jackie M Douglas,
Jacqueline D Mitchell,
James A Liubicich,
James C Maclaughlin,
James L Dunnie, Jr,
Janet B Gray,
Jason F Chancey,
Jason R Nickelsen,
Javier O Vega,
Jawaid Hamid,
Jeff M Uriarte,
Jeffrey D Sheppard,
Jeffrey P Raimondi,
Jennifer A Hood,
Jennifer C Adams,
Joe A Odom,
Joey V Quitania,
John D White,
John Stackman,
Johnson,
Jonathan W Chapman,
Jose A Lopez,
Jose Acruz Gonzalez,
José E Meléndez,
Jose M Cayuela,
Jose R Hernandez,
Joseph F Owens,
June P Page,
Junho Pak,
Justin A Boyd,
Kara D Dobbin,
Karen A Briggs,
Katherine Szestypalow,
Kellia N Hicks,
Kenitra D Hewitt,
Kenneth H Williams,
Kenneth R Merritt,
Kenny R Robinson,
Keri U Walker,
Kevin A Gonzalez,
Kham Phommachanh,
Lareese K Thomas,
Latorie S Jones,
Lauryl A Smith,
Lawrence Thomas,
LCDR Michael H Tollon,
Leah M Andrews,
Lillie D Witcher,
Linda A Gregory Duty,
Linda F Murphy,
LT Tamara J Henderson,
Luis A Dasta,
Luis Mburgos Medero,
Mancia R Walker,
Margaret L Bolte,
Margaret M Annes,
Maria Gutierrez Lugo, PhD,
Marianela Aponte Cruz,
Marie A Fadden,
Marie F Morin,
Matthew B Casale,
Mei Ou,
Merry Christie, PhD,
Metitia G Sanders,
Metitia M Gramby,
Michael A Charles,
Michael A Taylor,
Michael R Goga,
Michael Shanks, MS,
Michele Gottshall,
Michele L Obert,
Michelle D Haamid,
Mokhless A Ghaly,
Mra Davism,
Mra Mcculloughj,
Muna Algharibeh,
Muralidhara B Gavini, PhD,
Nadeem I Chaudhry,
Nancy E Fontaine,
Natalie Adan,
Nicholas L Hunt,
Nicole E Knowlton,
Paraluman S Leonin,
Parul M Patel,
Patrick C Klotzbuecher,
Paul E Stein,
Paul L Bellamy,
Paul W Moy,
Penny H Mccarver,
Peter E Baker,
Rachael L Cook,
Rafael Nevarez Nieves,
Rafeeq A Habeeb,
Randy L Clarida,
Rashonda N Rucker,
Raymond T Oji,
Rebecca Parrilla,
Reyes Candau Chacon, PhD,
Robert C Coleman,
Robert C Steyert,
Rochelle L Cross,
Roger F Zabinski,
Rory K Geyer,
Roy R Rinc,
Russell R Zablan,
Samina S Khan,
Sandra C Lawrence,
Sandra I Gaul,
Sangeeta M Khurana, PhD,
Sarah E Mcmullen,
Satheesh Thomas,
Scott N Lim,
Scott R Nichols, PhD,
Seneca D Toms,
Shanna N Purdy,
Sharna D Pratt,
Shawn E Larson,
Simone E Pitts,
Sixto M Mercado Rios,
Sonya M Edmonds,
Srinivas R Chennamaneni, PhD,
Stephanie A Slater, MS,
Stephen D Brown,
Steven D Dittert,
Steven D Kehoe,
Steven Frisbee,
Susan Alexander,
Susan M Taylor,
Susan O Oladeji,
Susanne M Richardson, MS RAC,
Tajah L Blackburn,
Tamil Arasu, PhD,
Tara L Stockton,
Tekalign Wondimu,
Teresa I Navas,
Theresa Kirkham (NMI),
Thomas J Arista,
Tina M Pawlowski,
Tomika L Bivens,
Tracy R Ball,
Truong Xuan Nguyen (Andy),
Tyrico K English,
Veronica Fuentes, MS,
Vicky C Stoakes,
Vilmary Negron Rodriguez,
Vincent M Williams,
Viviana Matta,
Vlada Matusovsky,
Wayne T Smith,
William A Warnick,
William J Leonard,
Yasamin Ameri,
Yiyue Zhang (nmi), PhD,
Yumi J Hiramine,
Yvette I Johnson,
Zada L Giles,
Zedong Dong
Reba A Gates's Documents
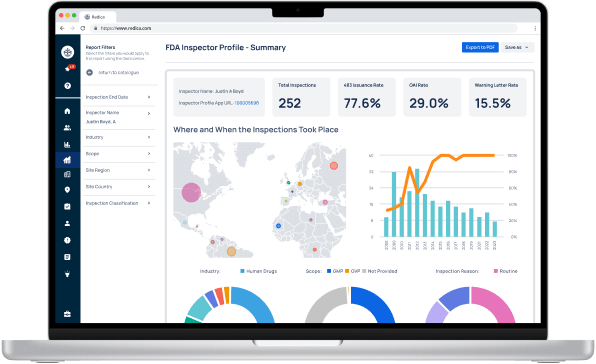
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more