FDA Investigator Doan T Nguyen, PharmD
Doan T Nguyen, PharmD has conducted inspections on 58 sites in 8 countries as of 26 Jul 2019. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
58
Last Inspection Date:
26 Jul 2019
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
India,
Canada,
United Kingdom of Great Britain and Northern Ireland,
United States of America,
Germany,
Romania,
Netherlands,
Puerto Rico
FDA Investigators that have inspected at least one site in common with Doan T Nguyen, PharmD:
Abby Lmozeke Baker,
Ademola O Daramola,
Agnes M Santori,
Aimee K Contreras,
Alan A Rivera,
Alan L Truong,
Alan P Kurtzberg,
Alexandra B Pitkin,
Alice S Tsao,
Alicia K Mckinsey,
Amalia C Himaya,
Anderson,
Andrew A Hoopes,
Angela E Glenn,
Angela Shepas,
Angelica M Hernandez,
Anh M Lac,
Anita Narula, PhD,
Ann L Demarco,
Anna Lazar,
Anthony C Warchut,
Anthony E Keller, RPh,
Anthony J Donato,
Arie C Menachem,
Azza Talaat,
Babajide Michael Osunsanmi,
Barry Cherney, PhD,
Bichsa T Tran,
Bijoy Panicker,
Binh T Nguyen,
Bo Chi, PhD,
Bonita S Chester,
Brenda K Alford,
Brenda Rivera,
Brenda W Uratani, PhD,
Brent T Hall,
Brett R Havranek,
Brian J Ryan,
Brien C Fox,
Brittany D Terhar,
Brooke K Higgins,
Bryan A Galvez,
Bryan L Mcguckin,
Burnell M Henry,
Candace S Tucker,
Carla J Lundi,
Carolina D Vasquez,
Carrie A Hughes,
Caryn M Mcnab,
Cassandra L Abellard,
Cassandra L Winters,
Cathleen A Carr Sharpe,
CDR Rochelle B Young, RPh, MSA,
Celena Ngo,
Charisse K Green,
Charles D Brown,
Charles L Larson,
Charles M Edwards,
Charles R Cote, RIC,
Cheron M Portee,
Chris A Sack,
Christian L Witkovskie,
Christina C Santos,
Christina V Santos,
Christopher Downey, PhD,
Christopher T Middendorf,
Cody J Alley,
Concepcion Cruz, Jr,
Dana D Carter, SR DDS,
Daniel W Cline,
Darren S Brown,
David G Whitman,
David L Miser,
David Y Hernandez,
Davinna Ligons, PhD,
Davis,
Deborah A Greco,
Debra I Love,
Devaughn Edwards,
Diane Cvan Leeuwen,
Diane L Raccasi,
Dina K West,
Dipesh K Shah,
Donald C Obenhuber, PhD,
Donna Ltartaglino Besone,
Dr. Ralph M Bernstein, PhD,
Dr. Robert C Horan, MD,
Dr. Zhihao Qiu (Peter), PhD,
Edmund F Mrak, Jr,
Eileen A Liu,
Eileen T Dupont,
Eliezar Ramos,
Elizabeth Ll Edwards,
Emmanuel Jramos Maldonado,
Eric M Mueller, PharmD,
Eric M Padgett,
Eric T Huebler,
Erika V Butler,
Erin D Mccaffery,
Esther C Broner, PhD,
Farhana Khan,
Felix Maldonado,
Finn A Slizza,
Frances Namuswe, PhD,
Freddy Ortiz Colon,
Gabriel Craig,
Gavin T Cua,
George Pyramides,
Gregson A Joseph,
Guerlain Ulysse,
H Ljamilla Selby,
Hala L Selby,
Haley H Seymour,
Haroon Vohra (NMI),
Hasan A Irier, PhD,
Helen B Ricalde,
Helen Verdel,
Henry E Carrillo,
Henry K Lau,
Heriberto Negron Rivera,
Herminio C Francisco,
Holly J Wilson,
Iris C Macinnes,
Isabel Y Espinosa,
Ivar J Sohn,
Jacob W Reynolds,
James A Barreto,
James A Lane,
James L Dunnie, Jr,
James P Stallings,
James R Fleckenstein,
Jee Chung, PhD,
Jeff M Uriarte,
Jeffrey M Watson,
Jeffrey P Raimondi,
Jennifer Gogley,
Jennifer M Gogley,
Jinnie Kokiatkulkij,
Joel Welch, PhD,
Joey V Quitania,
Jogy George,
John A Daubenspeck,
John A Gonzalez,
John D White,
John P Jorgensen,
John R Myung,
Jonathan K Dare,
Jorge E Martinez,
Jorge L Guadalupe,
Jose A Lopez,
Jose Acruz Gonzalez,
José E Meléndez,
Jose R Lopez,
Jose Rflores Veguilla,
Jose Velez,
Juanj N Wu,
Judy C Nepsa,
Julian C Hanson,
Julie D Bringger,
Junho Pak,
Justin A Boyd,
Ka L Wong,
Kaarin M Slotte,
Kalavati Suvarna, PhD,
Karen E D'orazio,
Kathleen D Culver,
Kathleen R Jones, PhD,
Kathryn E Ogger,
Kelley,
Kellia N Hicks,
Kelvin Cheung,
Kelvin X Sanders,
Kendra A Biddick,
Kevin A Gonzalez,
Kham Phommachanh,
Kim Lthomas Cruse,
Kimiko G Casuga,
Kurt A Brorson, PhD,
Lance Mde Souza, MBA,
Lata C Mathew, PhD,
Laura E Garcia,
Laura Fontan, MS,
Lauren R Collins,
Laurimer Kuilan Torres,
Lavender M Huskey,
LCDR Debra Emerson,
Leonard H Lavi,
Libia M Lugo,
Lilly O Barton,
Linda Thai,
Lisa L Flores,
Logan T Williams,
Lourdes Andujar,
Lucas B Leake,
Luis A Carrion,
Luis Mburgos Medero,
Marcellinus D Dordunoo,
Marcia B Williams,
Marcus F Yambot,
Marian E Ramirez,
Marianela Aponte Cruz,
Mariza M Jafary,
Mark Brunswick, PhD,
Mark E Parmon,
Marvin D Jones,
Mary E Storch,
Matt D Suedkamp,
Matthew A Johnson,
Matthew B Casale,
Maxyne T Lam,
Mei Chiung Huang (Jo),
Meisha R Sampson,
Meisha Waters,
Michael A Charles,
Michael D Garcia,
Michael E Maselli,
Michael L Casner,
Michael L Chasey,
Michael P Sheehan,
Michael R Klapal,
Michael S Araneta,
Michael Shanks, MS,
Michele L Obert,
Michele Perry Williams,
Michelle A Marsh,
Michelle Yclark Stuart,
Miguel A Martinez Perez,
Mihaly S Ligmond,
Milos Dokmanovic, PhD,
Minh D Phan,
Mohsen Rajabi Abhari, FDA,
Monica Commerford, PhD,
Mra Davism,
Mra Munizn,
Mra V Millarw,
Mujadala M Abdul Majid,
Muralidhara B Gavini, PhD,
Myriam M Sosa,
Nancy G Schmidt,
Natalie J Ayoub,
Naveen B Walker,
Nebil A Oumer,
Nianna C Burns,
Nicholas L Hunt,
Nicholas L Paulin,
Nicholas Obiri, PhD,
Nicola M Fenty Stewart,
Noreen Muñiz,
Omotunde O Osunsanmi,
Pankaj H Amin,
Paraluman S Leonin,
Paranthaman Senthamaraikannan,
Parul M Patel,
Patricia F Alberico,
Patrick C Klotzbuecher,
Paul A Bonneau,
Paul Mouris,
Peter E Baker,
Peter S Diak,
Phal K Chhun,
Philip F Istafanos, DMV, MS,
Prabhu P Raju,
Qing Joanna Zhou, PhD,
Rachel C Harrington,
Rafael Nevarez Nieves,
Rafeeq A Habeeb,
Raquel Gonzalez Rivera,
Reba A Gates,
Rebecca Parrilla,
Rebecca Rodriguez,
Regina T Brown,
Reyes Candau Chacon, PhD,
Richard Ledwidge (nmi), PhD,
Richard W Berning,
Richmond K Yip,
Robert D Tollefsen,
Robert Darius,
Robert J Martin,
Robert Jennings,
Roger F Zabinski,
Rona Leblanc, PhD,
Ronald L Koller,
Ronda Leblanc,
Rose Ashley,
Ross J Grigsby,
Rumany C Penn, PharmD,
Ruth A Williams,
Ruth Moore, PhD,
S Lori Brown, PhD MPH,
Saied A Asbagh,
Saleem A Akhtar,
Samina S Khan,
Samuel T Walker,
Sangeeta M Khurana, PhD,
Sanket N Patel,
Santos E Camara,
Sarah Arden,
Sarah E Mcmullen,
Scott K Zika,
Scott R Nichols, PhD,
Scott T Ballard,
Sean R Marcsisin,
Seema S Singh,
Selene T Torres,
Shaquenta Y Perkins,
Shirley J Berryman,
Sidney B Priesmeyer,
Sonia M Monges,
Sonia R Peterson,
Sonya M Edmonds,
Stacie A Woods,
Stanley Au,
Stephen J Mottola,
Steven Fong, MS, PhD,
Susan P Bruederle,
Susanna N Choi,
Taichun Qin, PhD,
Tamala P Magee,
Tamara M Kays,
Tamika White,
Tamil Arasu, PhD,
Terri L Dodds,
Thao T Kwan,
Thao X Tran,
Theresa Kirkham (NMI),
Thomas R Beilke,
Thuy T Nguyen, LCDR,
Timothy T Kapsala,
Torrey M Ward,
Toyin B Oladimeji,
Tracey L Harris,
Travis S Bradley,
Travis V Hull,
Troy A Huffman,
Truong Xuan Nguyen (Andy),
Ucheabuchi Cchudi Nwankwor,
Uttaniti Limchumroon (Tom),
Vaishali J Patel,
Veronica Fuentes, MS,
Victoria A Wagoner,
Vidya B Pai,
Vilmary Negron Rodriguez,
Virgilio F Pacio, CSO,
Walden H Lee,
Walter N Lange,
Wayne E Seifert,
William A Warnick,
Xiaokuang Lai, PhD,
Yanming An, PhD,
Yasamin Ameri,
Yen Tso Kuo,
Young M Choi, PhD,
Yumi J Hiramine,
Yvins Dezan,
Yvonne C Mcknight,
Yvonne C Wilkes,
Zachary L Stamm,
Zachary Ryan Harris,
Zerita White,
Zhigang Sun, PhD,
Zhong Li, PhD,
Ziyang Su, PhD
Doan T Nguyen, PharmD's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
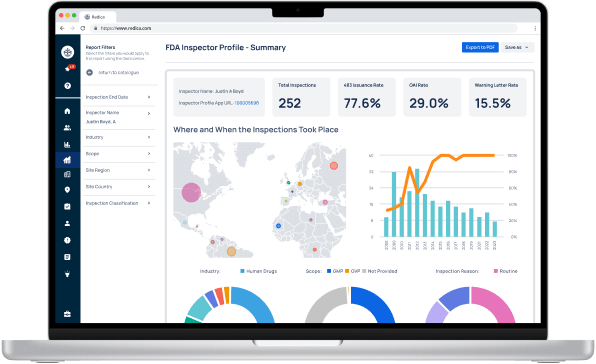
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more