FDA Investigator Joseph A Piechocki
Joseph A Piechocki has conducted inspections on 30 sites in 5 countries as of 10 Jun 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
30
Last Inspection Date:
10 Jun 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Italy,
India,
China,
Jordan
FDA Investigators that have inspected at least one site in common with Joseph A Piechocki:
Adam J Wilson,
Adam R Cooke,
Aditi S Thakur,
Adree N Anderson,
Alan L Truong,
Alan M Barker,
Alanna L Mussawwir Bias,
Alexandra A Carrico,
Alicia M Mozzachio,
Amanda M Dunneback,
Amy S Graf,
Ana Paulap Sandee,
Anders W Evenson,
Andrace L Deyampert,
Andrea D Swingle,
Andrew J Barrowcliff,
Angela E Glenn,
Anna M Brannen,
Anthony F Lorenzo,
Anthony J Ladner,
Anthony R Bucks,
April L Brown,
April L Young,
Arsen Karapetyan,
Art O Czabaniuk,
Aurora Miha Trifanov,
Azza Talaat,
Bei Y He,
Benjamin J Smith,
Benjamin W Anderson,
Bo Chi, PhD,
Brent M Dehaan,
Brian A Roelofs,
Brian D Nicholson,
Bruce H Mccullough,
Burnell M Henry,
Byungja E Marciante,
Caroline Strasinger,
Carolyn A Warren,
Carrie Ann Plucinski,
CDR Rochelle B Young, RPh, MSA,
Chad E Schmear,
Chad W Rice,
Charisse K Green,
Charles I Ann,
Charles L Zhou,
Cheryl A Clausen,
Cheryl A Grandinetti,
Christian D Lynch (CDL),
Christopher D Leach,
Christopher T Middendorf,
Colleen E Burke,
Constantin Y Philopoulos,
Corrine M Carter,
Courtney E Hillier,
Courtney R Tiegs,
Daniel J Brown,
David A Paterson,
David J Eide,
David L Miser,
David Perkins,
Dawn C Olenjack,
Debara R Reese,
Debara Reese,
Deborah M Trout,
Debra I Love,
Debra Lboyd Seale,
Denise L Burosh,
Dennis E Guilfoyle, PhD,
Devaughn Edwards,
Dilip Devineni, PhD,
Dina A Tallman,
Dipesh K Shah,
Donald C Obenhuber, PhD,
Dr. Carmelo Rosa,
Dr. Robert C Horan, MD,
Dr. Zhou Chen (nmi), MD PhD,
Edward E Lockwood (EEL),
Eileen A Liu,
Emest F Bizjak,
Emilie E Kahn,
Emily J Orban,
Eric M Mueller, PharmD,
Erika V Butler,
Franklin D Heisler,
Gayle S Lawson,
Gregory A Berg,
Gretchen Lf Trendel,
Hamet M Toure, PharmD MPH,
Hassan M Fakhreddine,
Hector A Carrero,
Howard Sklamberg,
Ian L Mcwilliams,
Ifueko Osemwota,
Ivan E Reyes,
Jacob G Lutz,
Jacqueline Mdiaz Albertini,
James R Evans,
James W Plucinski,
Janae D Price,
Janis R Armedoriz,
Jason F Chancey,
Jazmine N Brown,
Jazmine N Still,
Jean Blackston Hill,
Jeanne J Thai,
Jeffrey A Sommers,
Jeffrey D Meng,
Jennifer A Kemp,
Jennifer C Johnson,
Jennifer S Ness,
Jesse A Vazquez,
Jill J Tillman,
John M Seale,
John N Woodall,
Jonah S Ufferfilge,
Joohi Castelvetere,
Jose A Lopez,
Joseph A Morkunas,
Joseph R Strelnik,
Joseph T Madak,
Joslyn K Brunelle, PhD,
June P Page,
Justin A Boyd,
Kalavati Suvarna, PhD,
Karen M Montgomery (KMM),
Kelley L Clark,
Kenneth H Williams,
Kevin A Gonzalez,
Kham Phommachanh,
Kristina Fillmore,
Kristina Nastovski,
L'oreal F Walker,
Larry K Austin,
Laureen F Kononen,
Lauren E Sanger,
Lauren E Sexton,
Lauren Iacono Connors, PhD,
Lauren M Lilly, PhD,
Lauren N Howard,
Laurie Graham,
Leigh Anne Myers,
Lequita M Mayhew,
Lindsey Brown, PhD,
Lisa Hornback,
Lisa M Bellows,
Lisa R Hilliar,
Logan T Williams,
Luella J Rossi,
Luis A Dasta,
Maria C Iuga,
Marie A Fadden,
Marissa S Henning,
Marissa S Steinhagen,
Martha Sullivan Myrick,
Martin R Vowell,
Mary L Schuckmann,
Massod Rahimi, PhD,
Matthew B Casale,
Matthew M Schuckmann,
Megan A Haggerty,
Meisha R Sampson,
Meisha Waters,
Melina Lrodriguez Upton,
Mercy Oyugi,
Miah I Schneider,
Michael A Charles,
Michael Shanks, MS,
Michael Y Philopoulos,
Michele L Forster, PhD,
Michele L Glendenning,
Michele L Obert,
Michele Perry Williams,
Mihaly S Ligmond,
Monica E Murie,
Montgomery Montgomery, Karen M,
Muna Algharibeh,
Muralidhara B Gavini, PhD,
Myra K Casey,
Nailing Zhang,
Nancy L Rolli,
Neali H Lucas,
Nibin Varghese, MS,
Nicholas A Violand,
Nicholas J Presto,
Nicholas P Diorio,
Nicholas Z Lu,
Nicole E Knowlton,
Nicole S Williams,
Omotunde O Osunsanmi,
Ou Ma,
Paola S Barnett,
Parul M Patel,
Patricia A Cochran,
Patricia A Mcilroy,
Patricia H Dlugosz,
Patrick B Cummings,
Patrick C Klotzbuecher,
Patsy J Domingo,
Paul L Bellamy,
Pei I Chu,
Peng Zhou,
Peter E Baker,
Phung Thien Nguyen,
Prabhu P Raju,
Qiao Y Bobo,
Rafael Padilla,
Rafeeq A Habeeb,
Rajiv R Srivastava,
Ralph H Vocque,
Ramon E Martinez,
Raquel Gonzalez Rivera,
Raymond T Oji,
Rebecca E Dombrowski,
Richard Ledwidge (nmi), PhD,
Robert D Tollefsen,
Robert J Ham,
Robert M Barbosa,
Rohn R Robertson,
Rose Ashley,
Ross J Grigsby,
Rumany C Penn, PharmD,
Russell K Riley,
Saleem A Akhtar,
Salwa K Poole,
Sandra A Boyd,
Sandra Sejdinovic,
Sanket N Patel,
Sarah E Rhoades,
Sarah M Meng,
Sarah M Napier,
Scott B Laufenberg,
Shafiq S Ahadi,
Shalonda C Clifford,
Sharon K Thoma, PharmD,
Sherry G Bous,
Shirshendu K Deb, PhD,
Sidney B Priesmeyer,
Simone E Pitts,
Sixto M Mercado Rios,
Sneha S Patel,
Stephen C Mahoney,
Stephen D Brown,
Stephen D Eich,
Steven D Kehoe,
Susan D Yuscius,
Susan F Laska, MS,
Susan M Jackson,
Susan P Bruederle,
Suyang Qin,
Tamil Arasu, PhD,
Tara L Greene,
Tara L King,
Tenzin Jangchup,
Teresa I Navas,
That Q Dang,
Theodore N Sietsema,
Thomas J Arista,
Thuy T Nguyen, LCDR,
Tiara Nbrown Crosen,
Tina M Pawlowski,
Tina S Roecklein,
Vincent Thomas,
Walden H Lee,
Warren J Lopicka,
Wayne D Mcgrath,
Wayne E Seifert,
William A Warnick,
William D Tingley,
Xiaoshi N Wang,
Yangmin Ning,
Yehualashet A Gessesse,
Yiyue Zhang (nmi), PhD,
Yongmin Liu,
Yuanyuan Li,
Yumi J Hiramine,
Yvonne E Lozano,
Zhang Zhao, PhD
Joseph A Piechocki's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
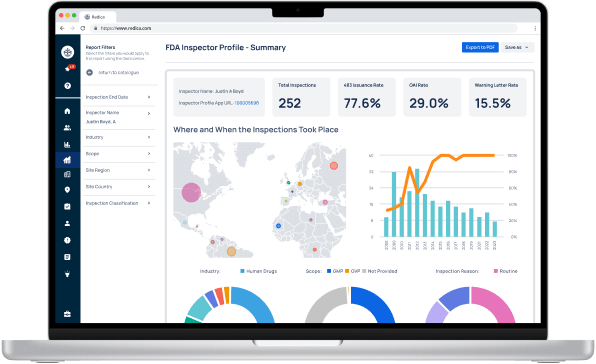
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more