FDA Investigator Sanket N Patel
Sanket N Patel has conducted inspections on 37 sites in 4 countries as of 25 Jun 2012. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
37
Last Inspection Date:
25 Jun 2012
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
Spain,
India,
United States of America,
France
FDA Investigators that have inspected at least one site in common with Sanket N Patel:
Abdul K Kamara,
Adam J Wilson,
Alan M Barker,
Alan M Roberts,
Alanna L Mussawwir Bias,
Amanda M Dunneback,
Amy S Graf,
Andrace L Deyampert,
Andre S Raw,
Anthony F Lorenzo,
Art O Czabaniuk,
Aurora Miha Trifanov,
Azza Talaat,
Bei Y He,
Benjamin J Smith,
Bonita S Chester,
Brent M Dehaan,
Brentley S Collins,
Brooke K Higgins,
Carolina D Vasquez,
Caroline H Le,
Caroline Strasinger,
Catherine V Quinlan,
Cathie S Marshall,
Charanjeet Jassal,
Charisse K Green,
Charles I Ann,
Charles L Zhou,
Charles M Edwards,
Christian D Lynch (CDL),
Christina Eggleston,
Constantin Y Philopoulos,
Courtney E Hillier,
Craig D Zagata,
Cynthia L Aycock,
Danial S Hutchison,
Daniel J Brown,
Daniel J Grabicki,
Daniel L Obrzut,
Darren S Brown,
Darren S Morgan,
David J Gasparovich,
Dawn C Olenjack,
Deborah M Trout,
Demitria J Xiradakis,
Dennis Cantellops Paite,
Dennis R Downer,
Doan T Nguyen, PharmD,
Eliezar Ramos,
Elizabeth J Rigoni,
Emest F Bizjak,
Emilie E Kahn,
Emily J Orban,
Emmanuel Jramos Maldonado,
Eric E Joneson,
Erin M Miller,
Freddy Ortiz Colon,
Gam S Zamil,
Gene D Arcy,
Geneve M Maxwell,
Geneve M Parks,
George G Calafactor,
Helen B Ricalde,
Ifueko Osemwota,
Ivis L Negron,
Jacob G Lutz,
Jacqueline Mdiaz Albertini,
James E Szelc,
James K Ireland,
James Norman,
James R Evans,
Janet L Bowen,
Jeannie M Melin,
Jeffrey A Sommers,
Jeffrey D Meng,
Jennifer A Kemp,
Jogy George,
Joohi Castelvetere,
Joseph A Piechocki,
Joseph T Madak,
Joshua M Adams,
Justin A Boyd,
Kathryn A Cutajar,
Kathryn A Guardiola,
Kathryn G Brown,
Kristina Mezin,
Kristina Nastovski,
Larry K Austin,
Laureen F Kononen,
Lauren E Sexton,
Lauren M Lilly, PhD,
LCDR Anastasia M Piliafas Brown,
Leslie A Paul,
Linda S Jozefiak,
Lindsey Brown, PhD,
Lindsey M Schwierjohann,
Lisa J Joseph,
Logan M Ebeling,
Luis Mburgos Medero,
Margaret M Annes,
Margaret N Klug,
Margaret N Persich,
Marlon K Turner,
Martha Sullivan Myrick,
Martin R Vowell,
Marvin D Jones,
Matthew B Casale,
Matthew J Gretkierewicz,
Meisha R Sampson,
Meisha Waters,
Miah I Schneider,
Michael A Charles,
Michael C Ireland,
Michael L Casner,
Michael Shanks, MS,
Michael Y Philopoulos,
Michele L Forster, PhD,
Michele Perry Williams,
Mihaly S Ligmond,
Mikhail V Ovanesov, PhD,
Myra K Casey,
Nancy L Rolli,
Neali H Lucas,
Nicholas E Boyd,
Omotunde O Osunsanmi,
Owens,
Paige E Shelborne,
Pankaj H Amin,
Patricia A Cochran,
Patricia H Dlugosz,
Patsy J Domingo,
Paula A Trost,
Philip A Klimkewicz,
Prabhu P Raju,
Pratik S Upadhyay, DDC,
Ralph A Erickson,
Raymond R Roy, PhD,
Rebecca E Dombrowski,
Reginald Walker,
Richard W Thornton,
Robert C Coleman,
Robert J Martin,
Robert M Barbosa,
Robert R Barbosa,
Ronald G Crawford,
Ronald J Kozara,
Rose Ashley,
Rozelle G Smith,
Ryan J Benedict,
Saied A Asbagh,
Salwa K Poole,
Samantha J Bradley,
Sangeeta M Khurana, PhD,
Sarah E Rhoades,
Sarah M Meng,
Sarah M Napier,
Sarah Rhoades,
Sargum C Sood,
Scott A Golladay,
Seema S Singh,
Shirshendu K Deb, PhD,
Sidney B Priesmeyer,
Sina Shojaee,
Sneha S Patel,
Stefen D Mcmillan,
Susan M Jackson,
Susan P Bruederle,
Susanna E Ford,
Tamil Arasu, PhD,
Thomas A Peter,
Thomas J Arista,
Thomas J Cosgrove,
Tina M Pawlowski,
Tina S Roecklein,
Tonnie L Carter,
Toyin B Oladimeji,
Tracey Asinjen Wiersma,
Tracey L Siebesma,
Twana R Chandler,
Vesa Vuniqi,
Vilmary Negron Rodriguez,
William D Tingley,
William G Nelson,
Yumi J Hiramine
Sanket N Patel's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| May, 2012 | FDA 483 | DDP Specialty Electronic Materials US 9, LLC HIMS - Form 483, 2012-05-18 |
| October, 2011 | FDA 483 | AstraZeneca Pharmaceuticals LP - Form 483, 2011-10-28 |
| September, 2011 | FDA 483 | Pharmacia & Upjohn Company LLC - Form 483, 2011-09-23 |
| May, 2010 | FDA 483 | Sixarp, LLC - Form 483, 2010-05-28 |
| March, 2010 | FDA 483 | Future Pak LLC - Form 483, 2010-03-10 |
| June, 2011 | FDA 483 | ENDO USA, Inc. - Form 483, 2011-06-08 |
| March, 2011 | FDA 483 | King Pharmaceuticals LLC - Form 483, 2011-03-16 |
| May, 2011 | FDA 483 | Lincare, Inc. dba Alert Medical - Form 483, 2011-05-05 |
| June, 2011 | EIR | Nutrition & Biosciences USA 1, LLC - EIR, 2011-06-16 |
| October, 2010 | FDA 483 | Corium Innovations, Inc - Form 483, 2010-10-29 |
| November, 2010 | FDA 483 | Kath Khemicals LLC - Form 483, 2010-11-24 |
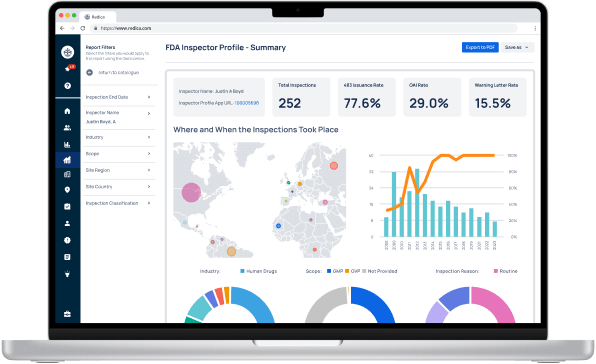
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more