FDA Investigator Lindsey M Schwierjohann
Lindsey M Schwierjohann has conducted inspections on 204 sites in 7 countries as of 07 Oct 2019. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
204
Last Inspection Date:
07 Oct 2019
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Italy,
Japan,
China,
Belgium,
Austria,
Taiwan
FDA Investigators that have inspected at least one site in common with Lindsey M Schwierjohann:
Ademola O Daramola,
Alan Escalona,
Alan M Barker,
Alec M O'neal,
Allison C Hunter,
Allison E Sincek,
Allison M Scheck,
Amanda M Dunneback,
Amber M Capello Jones,
Andrew I Carr,
Andrew J Barrowcliff,
Andrew M Kupper,
Andria L Kuhlman,
Angela E Glenn,
Anissa C Kelley,
Anna M Brannen,
Arie R Dyk,
Arsen Karapetyan,
Azza Talaat,
Bailey A Uetrecht,
Benjamin D Motsinger,
Benjamin D Shuler,
Bethany L Subel,
Blyss L Ketterer,
Brenda C Hamilton,
Brentley S Collins,
Brigitte K Hofmann,
Bryan J Love,
Burnell M Henry,
Byeongtaek Oh,
Caitlin L Almonrode,
Carlos H Ruiz,
Cassandra L Abellard,
Cassandra L Winters,
Catherine V Quinlan,
Charisse K Green,
Charles I Ann,
Charles L Zhou,
Charles M Edwards,
Chelsea J Falkowski,
Cherrie A Zachary,
Chris B Dedic, RS,
Christian D Lynch (CDL),
Christian L Witkovskie,
Christina Capacci Daniel, PhD,
Christina L Bigham,
Christine E Kelley,
Christopher T Middendorf,
Concepcion Cruz, Jr,
Constantin Y Philopoulos,
Courtney E Hillier,
Craig P Seaborn,
Craig T Rybus,
Cynthia M Goudeau,
Cynthia T Cain,
D Yvette Barnett,
Daisy E Ramos,
Danial S Hutchison,
Daniel J Brown,
Darren S Morgan,
David J Gasparovich,
David P Moorhead,
David S Jackson,
Dawn C Olenjack,
Dell S Moller,
Denise M Digiulio,
Diane R Mcdaniel,
Donald W Myrick,
Dongping Dai, PhD,
Donna F Campbell,
Dorathy M Eischen,
Doretha M Tonkins,
Dorothy P Kramer,
Dr. Gang Wang, PhD,
Dr. Jason R Caballero,
Dyvette Arline,
Edwin Melendez,
Eliezar Ramos,
Elisa A Nickum,
Elisa A Weckerling,
Elizabeth L Loreaux,
Elizabeth Ll Edwards,
Elizabeth P Kinsella,
Emest F Bizjak,
Emily J Orban,
Emma A Nesbit,
Felipe Rde Almeida,
Felix Maldonado,
Frank W Perrella,
Frederick M Lochner,
Gam S Zamil,
Gene D Arcy,
Geneve M Maxwell,
Geneve M Parks,
Genevieve E Ghand,
Geoffrey K Kilili,
George J Flynn,
Gerard T Schneider,
Gina M Brackett,
Gretchen N Kilby,
Hang N Guo,
Heather A Mccauley,
Helen B Ricalde,
Helen Verdel,
Holly J Wilson,
Hugh M Mcclure, II,
Isabel Y Espinosa,
Israel Santiago, OCM,
Ivis L Negron,
Jacob G Lutz,
Jacqueline Mdiaz Albertini,
James C Maclaughlin,
James G Marsh,
James K Ireland,
James Norman,
Jamie L Dion,
Janet L Bowen,
Jason D Werne,
Jason R Vidaurrie,
Javier O Vega,
Javonica F Penn,
Jazmine N Still,
Jeffrey A Sincek,
Jeffrey A Sommers,
Jeffrey D Meng,
Jennifer A Downie,
Jennifer A Pruden,
Jennifer L Gustavus,
Jennifer L Jager,
Jennifer L Sheehan,
Jennifer L Watson,
Jennifer Lalama,
Jennifer M Heitz,
Jeremiah D Beckwith,
Jerry J Corwin,
Jessica L Pressley,
Jessica M Wooten,
Jill J Tillman,
Jill R Loeliger,
Jill R Raezer,
John D Jaworski,
John E Russell, Jr,
Jon P Antoniou,
Jonathan A Ferguson,
Jonathan W Chapman,
Jose Acruz Gonzalez,
José E Meléndez,
Jose Martinez, Jr,
Jose Ohernandez Guzman,
Jose R Hernandez,
Joseph X Kaufman,
Joshua P Wireman,
Joshua S Hunt,
Justin A Boyd,
Karen M Cooper,
Katherine E Jacobitz,
Katherine Szestypalow,
Kathleen D Culver,
Kathryn J Mccarty,
Katie L Korchinski,
Keith J Mason, Jr,
Kelley L Clark,
Kellia N Hicks,
Kenneth E Felkley,
Kenneth H Williams,
Kham Phommachanh,
Kimberly A Joseph,
Kirk W Gaston,
Krista S Tuggle,
Krista T Ferry,
Kumar G Janoria,
Laureen M Geniusz,
Lauren N Howard,
Lauren N Smith,
Lauren R Collins,
Laurie Nelson,
Laurimer Kuilan Torres,
Leena Thomas,
Lequita M Mayhew,
Leslie A Paul,
Lianji Jin,
Lindsey K Giles Austin,
Lindsey S Fleischman,
Lisa M Marko,
Lisa R Hilliar,
Logan T Williams,
Lori A Carr,
Lori A Lahmann,
Luckner Jean Marie,
Luis A Dasta,
Lynnsey A Renn,
Malik S Qaiser,
Maney P Sturgill,
Mantai Z Mesmer,
Marcellinus D Dordunoo,
Marcus A Ray,
Maressa L Mills,
Marian E Ramirez,
Marianne Allen,
Maribeth G Grattan,
Maribeth G Niesen,
Marie F Morin,
Marijo B Kambere, PhD,
Marissa R Stough,
Mark E Parmon,
Martha Sullivan Myrick,
Mary B Sheets,
Mary E Storch,
Matt D Suedkamp,
Matthew B Casale,
Matthew H Pitts,
Matthew J Gretkierewicz,
Maureen F Alleman,
Meredith M Cobb,
Michael D Thatcher,
Michael E Campbell,
Michael E Clark,
Michael F Clark,
Michael J Schibi,
Michael K Larson,
Michael L Casner,
Michael P Sheehan,
Michael R Goga,
Michele L Forster, PhD,
Michele L Obert,
Michele Perry Williams,
Mihaly S Ligmond,
Minh D Phan,
Moseley Fulcher,
Mra Mcculloughj,
Mra Munizn,
Muna Algharibeh,
Myra K Casey,
Nadeem I Chaudhry,
Nancy Haggard,
Nancy L Neiger,
Nibin Varghese, MS,
Nicholas J Presto,
Nicholas L Paulin,
Nina Ni,
Nisha C Patel,
Noreen Muñiz,
Pamela A Kuist,
Paranthaman Senthamaraikannan,
Parul M Patel,
Patrick C Klotzbuecher,
Patsy J Domingo,
Patty P Kaewussdangkul,
Paula A Trost,
Peter E Baker,
Philip A Klimkewicz,
Philip F Istafanos, DMV, MS,
Philip S Woodward,
Phillip M Pontikos,
Pifer,
Prabhu P Raju,
Qiao Y Bobo,
Rafeeq A Habeeb,
Ralph A Erickson,
Ramon A Hernandez,
Raquel L Coleman,
Raymond R Roy, PhD,
Rebecca E Dombrowski,
Rebecca Rodriguez,
Renee N Easter,
Richard T Riggie,
Richard W Berning,
Richmond K Yip,
Robbie J Alleman,
Robert C Coleman,
Robert D Tollefsen,
Robert J Martin,
Robert M Barbosa,
Robert P Neligan,
Robert R Barbosa,
Robert Rodriguez,
Robert W Hudson,
Rohn R Robertson,
Ronald G Crawford,
Rosanna M Goodrich,
Rose Xu,
Roy C Stephens,
Saleem A Akhtar,
Samuel A Hamblin,
Sangeeta M Khurana, PhD,
Sanket N Patel,
Santos E Camara,
Sarah E Rhoades,
Sarah M Napier,
Sarah Rhoades,
Satheesh Thomas,
Scott A Golladay,
Shannon A Gregory,
Shannon D Fink,
Shannon F Underwood,
Shirley J Berryman,
Sina Shojaee,
Siobhan G Taylor,
Sixto M Mercado Rios,
Stephanie A Slater, MS,
Stephen J Kilker,
Stephen J Rabe,
Steven D Banks,
Steven P Eastham,
Susan M Jackson,
Susan M Taylor,
Susan P Bruederle,
Susan W Ting,
Sydney S Choi,
Taichun Qin, PhD,
Talmane J Fisher,
Tamara M Kays,
Tarun D Mehta,
Teresa I Navas,
Teresa K Kastner,
Theresa C Klaman,
Theresa Kirkham (NMI),
Thomas A Peter,
Thomas B Smith,
Thomas C Woods,
Thomas J Arista,
Thomas J Cosgrove,
Thomas J Prigge,
Thomas W Nojek,
Thunder N Dunkijacobs,
Tiara Nbrown Crosen,
Tonnie L Carter,
Tracey Asinjen Wiersma,
Tracey L Harris,
Travis V Hull,
Troy A Huffman,
Ucheabuchi Cchudi Nwankwor,
Uduak M Inokon,
Uttaniti Limchumroon (Tom),
Vaishali J Patel,
Vlada Matusovsky,
Walden H Lee,
Wen Ning Chan (Sally),
Wendy G Tan, PhD,
William A Warnick,
William D Tingley,
William J Leonard,
Ying Zhang,
Yumi J Hiramine,
Yvette Mlacour Davis,
Yvins Dezan,
Zachary A Bogorad
Lindsey M Schwierjohann's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
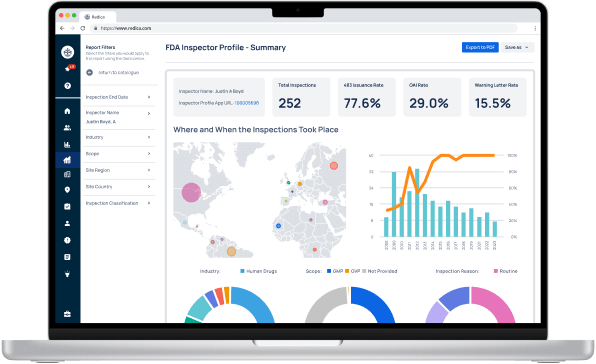
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more