FDA Investigator Scott E Norris
Scott E Norris has conducted inspections on 6 sites in 4 countries as of 19 Nov 2019. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
6
Last Inspection Date:
19 Nov 2019
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
France,
Ireland,
Netherlands
FDA Investigators that have inspected at least one site in common with Scott E Norris:
Alicia M Mozzachio,
Alla Kachko, PhD,
Amber D Brodas,
Anastasia I Offordile,
Anissa M Cheung,
Anita R Michael,
Ann L Demarco,
Ann Marie Montemurro,
Ann Marie Schofield,
Antonina Aydanian,
Audrey Thereset Uy,
Bijoy Panicker,
Brentley S Collins,
Brian D Nicholson,
Brian S Keefer,
Burnell M Henry,
CDR Donald Ertel,
CDR Jeremy L Wally, PhD,
Chao Ming Tsai,
Charles Jewell,
Christian D Lynch (CDL),
Christina J Sauder, PhD,
Christine A Harman, PhD,
Christopher J Adams,
Craig D Zagata,
Cynthia Jim, CSO,
Dave Hafner,
David J Hafner,
Deborah M Trout,
Debra J Bennett,
Debra L Pagano,
Demitria J Argiropoulos,
Demitria J Xiradakis,
Dennis E Guilfoyle, PhD,
Diane L Raccasi,
Dmitriy V Volokhov, PhD,
Eileen A Liu,
Ellen Huang,
Freyja V Lynn,
Gene D Arcy,
Hailin Wang (Sheena), PhD,
Hala L Selby,
Haruhiko Murata,
Helen B Ricalde,
J David Doleski,
Jacqueline Mdiaz Albertini,
Jacqueline S Warner,
James C Maclaughlin,
James M Mason,
James R Evans,
Jennifer L Bridgewater,
Jianyang Wang,
Jie He,
Joan A Loreng,
Joan Adamo,
Joanne M Hajoway,
John F Cipollo,
Joseph George,
Joyce A Rockwell,
Julianne C Mccullough,
Junho Pak,
Karen L Kosar,
Karyn M Campbell,
Kathleen B Mihalik,
Kathryn Carbone, MD,
Kelly N Kerr,
Kelly R Lewis,
Kenneth M Gordon,
Kevin A Gonzalez,
Kevin J Matthews,
Kevin P Foley,
Kristen D Evans,
Kristina L Conroy,
Larry K Austin,
Lata C Mathew, PhD,
Laurie P Norwood,
LCDR Margaret Edi Gennaro,
Lewis K Antwi,
Linda Thai,
Lisa M Bellows,
Lisa M Feola,
Lori Peters,
LT John M Mastalski,
Madushini Dharmasena, PhD,
Maria Joselopez Barragan, PhD,
Marian E Major, PhD,
Marian Majors, PhD,
Marjorie A Shapiro, PhD,
Marsha W Major,
Martha T Olone,
Mary Davis Lopez,
Mate Tolnay,
Matthew A Spataro,
Michael Curbarg,
Michael Gurbarg,
Michele L Forster, PhD,
Michele L Obert,
Mihaly S Ligmond,
Milan S Blake,
Nancy T Waites,
Nawab A Siddiqui,
Nicole K Trudel,
Omotunde O Osunsanmi,
Pankaj H Amin,
Patrick C Klotzbuecher,
Paul Mouris,
Paula A Trost,
Perry T Nichols,
Prabhu P Raju,
Ramon E Martinez,
Rebecca K Olin,
Richard Heath Coats,
Richard W Thornton,
Robert B Shibuya, MD,
Robert D Tollefsen,
Robert Jennings,
Robin Levis, PhD,
Ronit Jolles Mazor,
Rose Ashley,
Sarah B Tanksley,
Sheila Dreher Lesnick,
Shuang Tang,
Sidney B Priesmeyer,
Simone E Pitts,
Stephanie Mangigian, MS/OSH, RN,
Stephen D Brown,
Steven A Rubin,
Susan F Laska, MS,
Susan M Jackson,
Tammy L Chavis,
Tina S Roecklein,
Tracy R Ball,
Travis S Bradley,
Vlada Matusovsky,
Wayne E Seifert,
Wei Wang, PhD,
Xiao Wang,
Xu Michael Di, PhD,
Yvins Dezan
Scott E Norris's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| September, 2017 | FDA 483 | Sanofi Pasteur - Form 483, 2017-09-27 |
| September, 2019 | EIR | Merck Sharp & Dohme LLC - EIR, 2019-09-20 |
| September, 2018 | FDA 483 | Pfizer Ireland Pharmaceuticals - Form 483, 2018-09-18 |
| October, 2010 | FDA 483 | Wyeth Pharmaceutical Division of Wyeth Holdings LLC - Form 483, 2010-10-01 |
| April, 2016 | FDA 483 | SynCo Bio Partners Bv - Form 483, 2016-04-06 |
| November, 2019 | FDA 483 Response | Wyeth Pharmaceutical Division of Wyeth Holdings LLC - Form 483R, 2019-12-18 |
| September, 2019 | FDA 483 Response | Merck Sharp & Dohme LLC - Form 483R, 2019-10-07 |
| September, 2019 | FDA 483 | Merck Sharp & Dohme LLC - Form 483, 2019-09-20 |
| November, 2019 | FDA 483 | Wyeth Pharmaceutical Division of Wyeth Holdings LLC - Form 483, 2019-11-26 |
| November, 2019 | EIR | Wyeth Pharmaceutical Division of Wyeth Holdings LLC - EIR, 2019-11-26 |
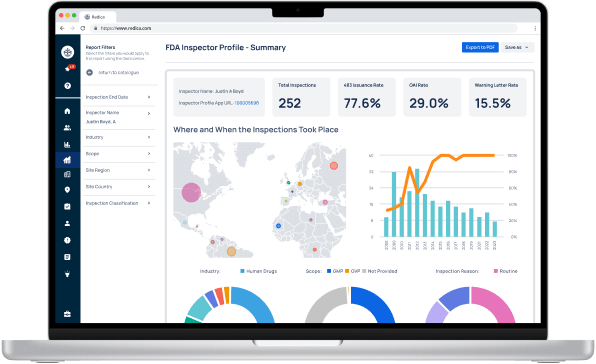
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more