FDA Investigator Lewis K Antwi
Lewis K Antwi has conducted inspections on 132 sites in 6 countries as of 21 Mar 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
132
Last Inspection Date:
21 Mar 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
France,
Belgium,
United Kingdom of Great Britain and Northern Ireland,
Italy,
Denmark
FDA Investigators that have inspected at least one site in common with Lewis K Antwi:
Abby Lmozeke Baker,
Abdur Razzaque,
Adam J Taylor,
Aditi S Thakur,
Aikaterini Alexaki,
Alan L Truong,
Alanna L Mussawwir Bias,
Amanda L Baskin,
Amanda S Zorn,
Amina Bashir,
Andrea D Swingle,
Andrew A Hoopes,
Andrew Byrnes, PhD,
Andrew J Barrowcliff,
Angela J Alexander,
Angela K Shen,
Angela S Whatley,
Anissa M Cheung,
Anthony R Bucks,
Antonio M Hampton,
Arie C Menachem,
Ashley A Mutawakkil,
Ava M Bowman,
Barbara A Rusin,
Barbara G Peoples,
Benjamin J Smith,
Bernard P Heidt,
Bharat Khurana,
Bo Chi, PhD,
Bradley J Maunder,
Brandi N Mcgrady,
Brenda C Hamilton,
Brenda L Reihing,
Brenda W Uratani, PhD,
Brent T Hall,
Brentley S Collins,
Brett R Havranek,
Brian J Davis,
Brittany D Terhar,
Brittany M Lowe,
Bryan A Galvez,
Bryan L Mcguckin,
Bryce E Mansfield,
Burnell M Henry,
Calvin K Cook,
Camilla S Smith,
Candace Y Gomez Broughton,
Carl A Huffman, III,
Carmen Y Fisher,
Caryn M Everly,
Caryn M Mcnab,
Cassandra Overking,
Catherine J Laufmann,
Catherine V Quinlan,
CDR Kimberly Y Martin,
Charles L Zhou,
Charles M Spyr,
Chava Kimchi Sarfaty, PhD,
Christian D Lynch (CDL),
Christine A Harman, PhD,
Christine W Twohy,
Clotia Cabbey Mensah,
Cody D Rickman,
Constantin Y Philopoulos,
Corrine M Carter,
Crystal M Young Lewis,
Cynthia Jim, CSO,
Damaris Y Hernandez,
Dana D Carter, SR DDS,
Danial S Hutchison,
Daniel J Lahar,
David J Eide,
David L Miser,
David S Cho, PhD,
Dawn C Olenjack,
Debara R Reese,
Deborah A Nebenzahl,
Deborah M Trout,
Debra Lboyd Seale,
Denise L Burosh,
Destry M Sillivan,
Dina K West,
Donald C Obenhuber, PhD,
Donald L Myers,
Dr. Robert C Horan, MD,
Eboni S Funderburk,
Edmund F Mrak, Jr,
Edwin Melendez,
Eileen A Liu,
Elisa M Fleming,
Elizabeth E Hart (ASCP), MT,
Ellen P Madigan,
Elyshia Q Danaher,
Emest F Bizjak,
Eric M Mueller, PharmD,
Eric M Padgett,
Eric Zhu,
Erika V Butler,
Erin C Hill,
Erma Zaimova,
Everard A Irish,
Farhana Khan,
Felix Maldonado,
Frederick M Lochner,
Gabryelle J Solverud,
Garrad R Poole,
Gene D Arcy,
Gerald B Seaborn, Jr,
Ginger M Sykes,
Grace E Mcnally,
Gwyn G Dickinson,
Hanna L Potter,
Hector A Carrero,
Helen B Ricalde,
Helen Y Saccone,
Howard A Burmester,
Humera T Khan,
Ifueko Osemwota,
Ingrid Y Johnson,
J David Doleski,
Jacek Cieslak, PhD,
Jacqueline Mdiaz Albertini,
James A Lane,
James C Maclaughlin,
James D Planchon,
James I Giefer,
James R Evans,
James R Fleckenstein,
James R Montero,
Jamie L Dion,
Janae D Price,
Janet B Abt,
Janet L Bowen,
Jazmine N Still,
Jean Lhu Primmer,
Jeanne J Chiu,
Jeanne J Thai,
Jeanne M Morris,
Jennifer A Hickey,
Jennifer A Jones,
Jennifer R Johnson,
Jessica L Peterson,
Jessie E Morris,
Jianyang Wang,
Jie He,
Joan A Loreng,
John A Iwen,
John D Finkbohner, PhD,
John M Mcinnis,
John M Mclnnis,
John S Hartford,
Joseph R Lambert,
Joseph R Strelnik,
Joyce A Rockwell,
Juan A Morales,
Judith A Jankowski,
Justin A Boyd,
Kaarin M Slotte,
Kalavati Suvarna, PhD,
Karen Emasley Joseph,
Karen M Cooper,
Karen M Labounty,
Kathleen S Tormey,
Kecia M Smith,
Kelley L Clark,
Kelly K Nachtigal,
Ken L Bowers,
Kevin A Gonzalez,
Kevin D Kallander,
Kevin J Matthews,
Kimberley A Hoefen,
Kip J Hanks,
L Norwood,
L'oreal F Walker,
Laura Fontan, MS,
Laureen F Kononen,
Laurel A Beer,
Lauren A Crivellone,
Lauren M Lilly, PhD,
Laurie Graham,
Laurie P Norwood,
LCDR Debra Emerson,
LCDR Ismael Olvera, IV,
Lee Terry Moore,
Leigh Anne Myers,
Lequita M Mayhew,
Lesley Maep Lutao,
Lily Y Koo,
Linda Thai,
Lisa M Thursam,
Lisa P Oakes,
Lori A Holmquist,
Lori Peters,
Lynn L Wong,
M Pedgen,
Maira P Brading,
Malgorzata G Norton, PhD,
Marc A Jackson, Jr,
Marcus L Head,
Margaret A Smithers,
Margaret Torres Vazquez,
Margarita Santiago,
Marianne Allen,
Marie B Buen Bigornia,
Marijo B Kambere, PhD,
Marion D Wimberly, Jr,
Marion Michaelis,
Marion W Nadeau,
Mariza M Jafary,
Mark A Elengold,
Mark I Kaspar,
Marlene G Swider,
Marlon K Turner,
Mary E Storch,
Mary Jeanet Mcgarry,
Maryann Ruff,
Matthew J Gretkierewicz,
Matthew J Sienko,
Matthew M Schuckmann,
Matthew R Dionne,
Matthew R Sleeter,
Megan A Haggerty,
Meisha R Sampson,
Meisha Waters,
Melissa D Kalik,
Melissa J Holz,
Michael A Blaine,
Michael D Robinson,
Michael I Gorman,
Michael J Kuchta,
Michael P Sheehan,
Michael R Goga,
Michael Y Philopoulos,
Michele L Forster, PhD,
Michele L Obert,
Michele Perry Williams,
Michelle Yclark Stuart,
Mihaly S Ligmond,
Minerva Rogers,
Misty D Harvey,
Monica M Mcclure,
Monique M Brooks,
Mra Munizn,
Muna Algharibeh,
Myra K Casey,
Nancy A Saxenian Emmons,
Nancy G Schmidt,
Nancy L Neiger,
Natalya M Ananyeva,
Nerizza B Guerin,
Nicholas Obiri, PhD,
Nicole K Trudel,
Noreen Muñiz,
Obinna R Echeozo,
Omotunde O Osunsanmi,
Pankaj H Amin,
Patricia A Mcilroy,
Patricia D Stahnke,
Patricia F Hughes, PhD,
Patricia H Dlugosz,
Patrick C Klotzbuecher,
Paul A Bonneau,
Paul M Kawamoto,
Paula A Trost,
Pawan K Jain,
Pete Amin (Pankaj),
Philip E Ake,
Prabhu P Raju,
Priscilla M Pastrana,
Ralph H Vocque,
Ramon E Martinez,
Randa Melhem, PhD,
Ranjani Prabhakara,
Rashmi Rawat, PhD,
Rebecca E Dombrowski,
Rebecca K Olin,
Reginald Walker,
Richard H Penta,
Richard L Rutherford,
Ricki A Chase,
Rita K Kabaso,
Robert C Coleman,
Robert J Ham,
Robert J Martin,
Robert J Nesselhauf,
Robert Jennings,
Robert M Barbosa,
Roberta W Cunningham,
Robin Levis, PhD,
Roman T Drews, PhD,
Ronit Jolles Mazor,
Rose Ashley,
Russell K Riley,
Samuel W Labinjo,
Santiago Gallardo Johnson,
Sarah A Palmer,
Sarah E Rhoades,
Satheesh Thomas,
Scott A Golladay,
Scott E Norris,
Scott R Nichols, PhD,
Scott T Ballard,
Sean R Marcsisin,
Shafiq S Ahadi,
Sharon I Gundersen,
Sharon K Thoma, PharmD,
Sherri J Jackson,
Shirley J Berryman,
Sidney B Priesmeyer,
Simone E Pitts,
Stacey S Degarmo,
Stephanie Mangigian, MS/OSH, RN,
Stephen R Souza,
Steven A Rubin,
Steven C Madzo,
Steven D Kehoe,
Steven E Bowen, PhD,
Steven P Donald,
Steven P Eastham,
Susan F Laska, MS,
Susan M Jackson,
Susan M Miller,
Susan M North,
Susan P Bruederle,
Susan T Hadman,
Susan Yu,
Susanna E Ford,
Svetlana A Shestopal,
Tania Y Hall,
Tara L Breckenridge,
Tara L Greene,
Telicia R White,
Thai D Truong,
Theressa B Smith,
Thomas J Arista,
Tiara Nbrown Crosen,
Tracy R Ball,
Travis S Bradley,
Unnee Ranjan,
Uruaku A Obasi,
Uttaniti Limchumroon (Tom),
Vera M Anderson,
Vickie J Kanion,
Vijaya L Simhadri,
Viviana Matta,
Viviana R Ramirez,
Walsworth,
Wayne E Seifert,
Wayne W Grundstrom,
Wei Wang, PhD,
William F Lagud, Jr,
William R Brubaker,
Xiaojun Yan,
Xiaokuang Lai, PhD,
Xiaoping Guan,
Zhong Li, PhD,
Zhongren Wu,
Zi Qiang Gu
Lewis K Antwi's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
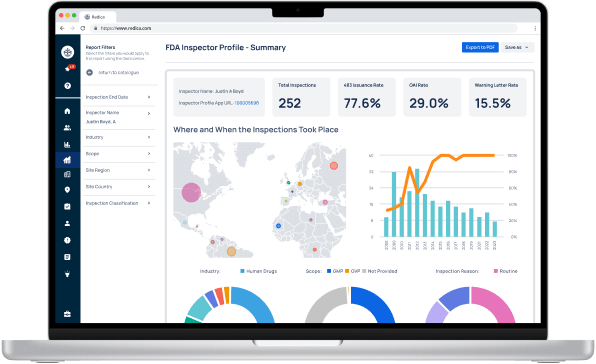
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more